Alzheimer’s Treatment. Next year might bring a major game-changer for folks dealing with Alzheimer’s in Britain. Some groundbreaking drugs designed to slow down the disease might finally be up for grabs.
Breaking Ground in Treatment
The US and Japan recently gave the nod to the first of these drugs called lecanemab. It’s already in action there. Another one, donanemab, is queuing up for approval. And guess what? The UK might give them the green light next year.
This news has got people crossing their fingers, hoping that after ages of trying, we might just crack the code on handling dementia in the UK. Right now, about a million folks here are wrestling with it, and by 2040, that number might shoot up to 1.7 million. That’s a pretty worrying picture. Last year, dementia claimed 66,000 lives in England and Wales, becoming the top cause of death. And two-thirds of those cases were Alzheimer’s.
A Hopeful Leap Forward
Until now, doctors could only offer meds to help folks manage their symptoms. But these new drugs? They could actually tackle the root cause. David Thomas from Alzheimer’s Research UK says they can slow down Alzheimer’s for about six months to a year. They’re not magical cures, but they’re a big step in the right direction after years of research.
Cath Mummery, a neurologist at University College London, agrees. She’s stoked that finally, there’s something promising on the horizon.
Targeting the Culprit
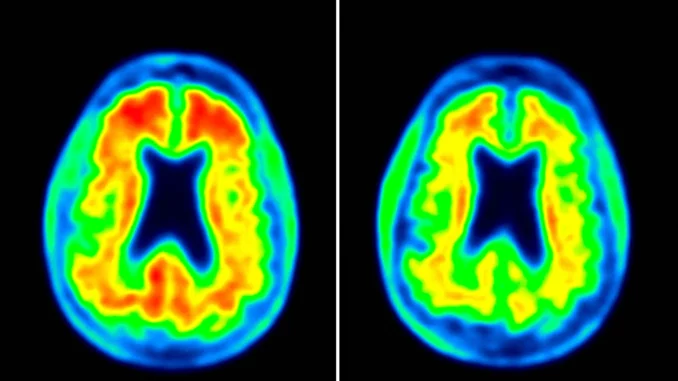
Alzheimer’s kicks off with a protein buildup called amyloid in the brain. The catch? Symptoms may not show up for decades after this protein starts piling up. Scientists have been hustling for over 20 years, trying to stop amyloid from forming these sticky plaques in hopes it’ll slam the brakes on the disease.
Lecanemab by Eisai in Japan and donanemab by Eli Lilly in the US are the first meds to pull this off. But here’s the thing: they only slow it down. They can’t hit the brakes entirely.
Challenges Ahead
Before these drugs hit shelves, they gotta pass the UK’s safety and effectiveness tests by the Medicines & Healthcare products Regulatory Agency (MHRA). Then, it’s up to the National Institute for Health and Care Excellence (Nice) to decide if they’re worth the price.
But here’s the rub: they ain’t cheap. Lecanemab costs about $25,000 (£19,700) a year and needs regular IV infusions. It’s a hassle for health services too, finding space and time to treat folks.
Diagnosis Dilemma
Spotting dementia, especially in its early stages, ain’t a walk in the park. Most cases start with GPs and then head to memory clinics for tests. But the wait? Sometimes up to two years on average. That’s a long time to twiddle your thumbs.
On top of that, confirming Alzheimer’s and other dementia types usually involves pen-and-paper tests, lumbar punctures, and brain scans. Roughly 65% of cases get confirmed this way. But a third? They slip through the cracks. And here’s the kicker: treatments, including these new drugs, only happen if you’re diagnosed.
The Pain of Delayed Diagnosis
Eleanor Mackenzie-Smith’s dad started showing Alzheimer’s signs when she was just 11. It took a whopping ten years and four tests to nail down his diagnosis. It’s a story too many families know firsthand, waiting helplessly as dementia takes hold without a diagnosis or access to treatments.
Looking Ahead
Doctors are eyeing blood tests as a quick and reliable way to spot the disease. But that’s still a few years away. For now, the NHS needs a serious upgrade to speed up diagnoses. Early detection is key, catching the disease when it’s just starting to stir.
Future Hopes
Scientists are optimistic about new ways to tackle dementia more effectively. They’re working on getting drugs past the blood-brain barrier, a real hurdle in treating brain issues. It’s all still in the works, though, and it’ll take time.
For now, it’s a step in the right direction, but there’s still a long way to go in dealing with what’s happening in our brains. It’s a tough challenge, but an important one to tackle.